Alzheimer’s Disease
Key Takeaways
Alzheimer’s disease is the most common type of dementia, progressing slowly and impacting memory, reasoning, and language.
Diagnosis has advanced with biomarkers, neuroimaging, and lab tests, but there is still no cure.
Symptoms progress through stages: pre-clinical, mild cognitive impairment, and dementia (mild, moderate, severe).
ICD-10-CM codes distinguish Alzheimer’s by age of onset (early vs. late) and exclude coding with senile dementia.
FDA recently approved new drugs (Lecanemab-imb, Donanemab-azbt) to slow disease progression, requiring precise HCPCS coding for claims.
According to the National Institute of Health, “Dementia is a general term used to describe a significant decline in cognitive ability that interferes with a person's activities of daily living. Alzheimer disease (AD) is the most prevalent type of dementia, accounting for at least two-thirds of cases in individuals aged 65 and older.” Alzheimer’s disease is considered to be a neurocognitive condition. The onset is slow, and as it progresses over time affecting memory, comprehension, attention, reasoning, judgement and language. Despite CDC data showing Alzheimer’s is ranked as the 7th leading cause of death, the condition itself does not directly cause death. The presence of related deficits and complications incurred from Alzheimer’s can lead to a patient’s demise.
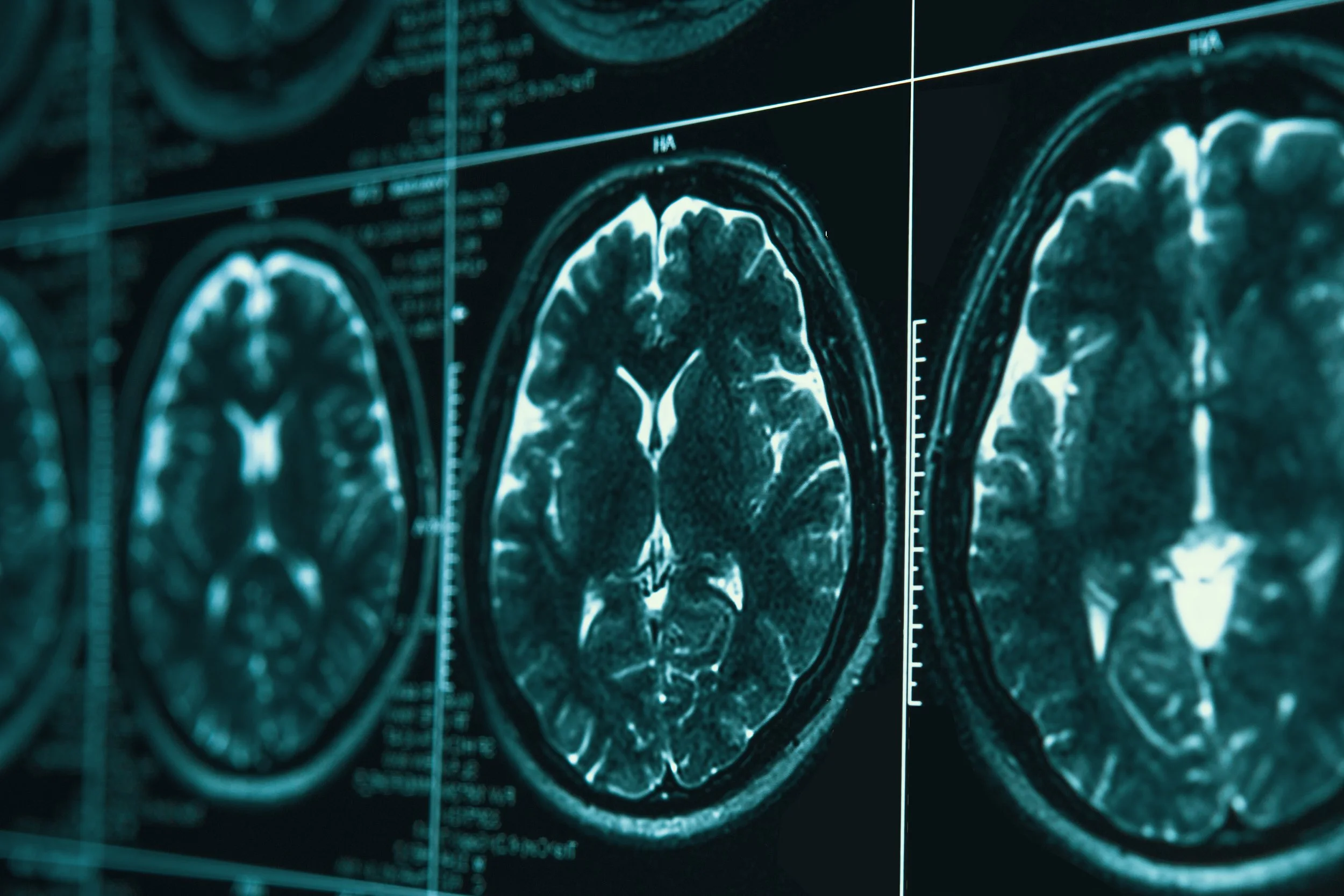
Over the past 10 years new options have emerged for diagnosing Alzheimer’s such as: the development of biomarkers that include neuroimaging markers used through amyloid and tau PET scans, Cerebral spinal fluid, and plasma markers (amyloid, tau, and phosphor-tau levels). There is no cure for Alzheimer’s Disease but there are new treatments emerging to lessen the symptoms and slow the progression of the disease.
The symptoms of AD will vary based on the stage of the disease. Stages are assigned based on the levels of cognitive decline and disability. The stages are Pre – clinical or presymptomatic stage, mild cognitive impairment, and dementia. The dementia stage is further sub divided into mild, moderate, and severe. These stages do not match the diagnostic criteria in DSM -5 for AD. For coders the authoritative resource is provider documentation in the clinical record.
ICD 10 CM Instructions for Alzheimer’s coding shows the following codes should be added as appropriate.
ICD 10 CM excludes diagnoses for senile dementia from being coded with Alzheimer’s disease.
CD 10 CM distinguishes between the age of onset for Alzheimer’s Disease. ICD 10 CM code G30.0 - Alzheimer's disease with early onset, refers to cases of Alzheimer’s that have an onset prior to age 65. Usually this happens after age 50 , as it is extremely rare for it to occur between the age of 30 and 40. Only 5-6% of patients are diagnosed with early onset Alzheimer’s. G30.1 is applied for Alzheimer’s that is diagnosed after age 65. When there is no information as to the age of onset of Alzheimer’s Disease G30.8 is the most appropriate code to apply.
The FDA has recently approved 2 new medications to slow Alzheimer’s Disease progression. HCPCS code J1074 Injection, Lecanemab-imb, 1mg, and J0175 Injection Donanemab- azbt, 2mg were added in 2023 and 2024 respectively. Make sure you accurately translate the dosage to the claim form based on the administered amount. Donanemab-azbt is listed as a unit does of 2mg. According to the manufacturer it is supplied in a single dose vial of 350 mg/20 ml and requires reconstitution with Normal Saline Solution (NSS - 0.9% sodium chloride). The resulting final concentration range is from 4 mg/ml to 10 mg/ml. The initial recommended dosage is 700 mg. via intravenous (IV) infusion over 30 minutes every four weeks for the first three doses. The dose doubles with dose 4 to 1,400 mg. every four weeks. This dosing would result in 350 units of HCPCS code J0175 for the 700 mg dose and 700 units for the 1400 mg. dose..
Some providers are reluctant to assign a definitive diagnosis of Alzheimer’s in a living patient. According to the National Institute of Aging through the National Institute of Health, “before the early 2000s, the only sure way to know whether a person had Alzheimer’s disease was through autopsy, a procedure that is performed after death.” Now lab and imaging tests are available to help providers see biological signs of the disease, or biomarkers, in a living person. An example is a blood test to measure levels of beta-amyloid, a protein that accumulates abnormally in the brains of people with Alzheimer’s disease. https://www.nia.nih.gov/health/alzheimers-symptoms-and-diagnosis/how-alzheimers-disease-diagnosed
Diagnosing remains a provider prerogative. Queries can be generated as needed to validate data and diagnoses.
ELEVATE MEDICAL SOLUTIONS: YOUR TRUSTED PARTNER MEDICAL CODING
At Elevate, we’re dedicated to helping medical coders like you excel. Stay ahead of the curve by subscribing to our blog for the latest resources, industry insights, and exclusive webinars where you can earn AHIMA-approved CEUs—all for free.
Join Our Community Today!